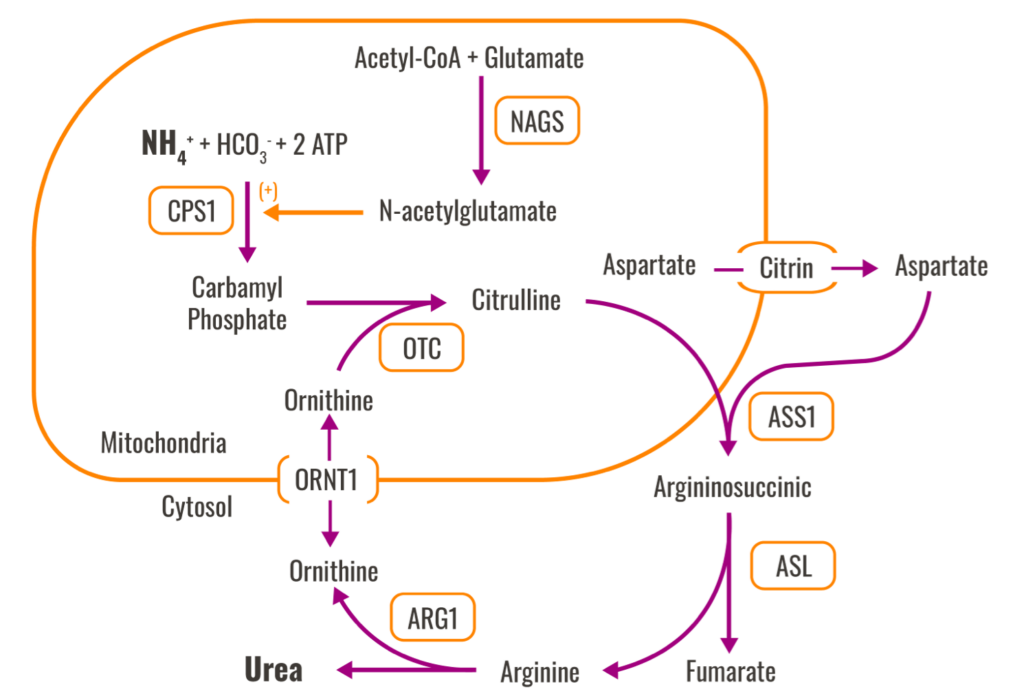
Disorders of the urea cycle

UCD symptoms often develop at home, after a newborn has been discharged, and may not be readily recognized by the family or primary care physician.1

UCD symptoms often develop at home, after a newborn has been discharged, and may not be readily recognized by the family or primary care physician.1

Molecular genetic testing is the definitive diagnostic test to confirm all types of UCDs.1

Molecular genetic testing is the definitive diagnostic test to confirm all types of UCDs.1
References
- Ah Mew N, Simpson KL, Gropman AL, Lanpher BC, Chapman KA, Summar ML. Urea cycle disorders overview [updated June 22, 2017]. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. University of Washington; 1993-2022. Accessed March 20, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1217/
- McGuire PJ, Lee HS, members of the Urea Cycle Disorders Consortium, Summar ML. Infectious precipitants of acute hyperammonemia are associated with indicators of increased morbidity in patients with urea cycle disorders. J Pediatr. 2013;163(6):1705-1710.
- Summar M. Current strategies for the management of neonatal urea cycle disorders. J Pediatr. 2001;138(suppl 1):S30-S39.
- Kölker S, Garcia-Cazorla A, Valayannopoulos V, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J Inherit Metab Dis. 2015;38(6):1041-1057.
- Gardeitchik T, Humphrey M, Nation J, Boneh A. Early clinical manifestations and eating patterns in patients with urea cycle disorders. J Pediatr. 2012;161(2):328-332.